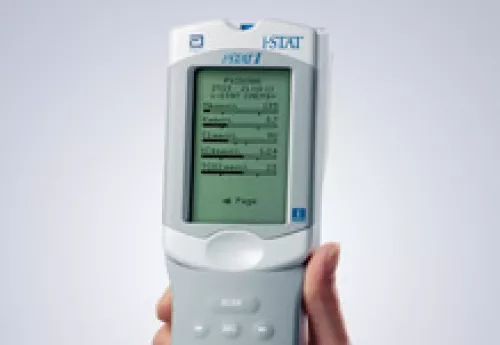
TRACK-TBI has partnered with Abbott to test its prototype TBI point-of-care device. The i-STAT handheld blood analyzer and blood test under development can be used at the patient’s bedside to detect elevated levels of TBI biomarkers like GFAP and UCHL-1. To date, the study has enrolled more that 1,000 participants across 10 TRACK-TBI NET sites. Participants have been enrolled that present with a suspected TBI (according to ACRM criteria) within 12 hours of the injury and who have a CT scan ordered. A blood draw is collected within 0-12 hours AND within 12-24 hours as well as various outcome assessments at enrollment and a full outcome battery at 2W, 6W, 3M, and 6M. There will also be an MRI and an additional blood draw each at 2W and 6M.